In a bid to save some time given looming grant application deadlines and overdue paper revisions, I’ve opted to reproduce a nice little discussion about how we define ‘species’ in a biodiversity sense. This is a great little synopsis of the species concept by Professor Colin Groves of the Australian National University that aired on ABC Radio National‘s Ockham’s Razor show hosted by Robyn Williams. This is an important discussion because it really dictates how we measure biodiversity, and more importantly, how we should seek to restore it when ‘degraded’. The full transcript can be viewed here, and you can listen here. Below I reproduce the relevant bits of the essay.
 Species, in the words of the great evolutionary biologist George Gaylord Simpson, are lineages evolving separately from others, each with its own unitary evolutionary role and tendencies. They are the units of biodiversity. Everybody uses the term, with greater or lesser degrees of precision, but even biologists, I regret to say, often use it without actually defining what they mean.
Species, in the words of the great evolutionary biologist George Gaylord Simpson, are lineages evolving separately from others, each with its own unitary evolutionary role and tendencies. They are the units of biodiversity. Everybody uses the term, with greater or lesser degrees of precision, but even biologists, I regret to say, often use it without actually defining what they mean.
It was the great zoologist Ernst Mayr who in 1940 offered the best known definition: ‘A species is a group of actually or potentially interbreeding natural populations which is reproductively isolated from other such groups’. He called this the Biological Species Concept.
This definition of species, still widely accepted, has frequently been misinterpreted as meaning that ‘different species cannot interbreed’. It does not say this. In the first place, it refers to species as ‘natural populations’. It is referring to what happens in a state of nature, not what happens in zoos or in domestic animals. For example, lions and leopards, which although closely related are usually recognised as different species, live in the same habitats in Africa and India and, as far as I know, no authenticated hybrids are known from the wild. But in zoos, hybrids have been bred successfully.
Then there is the question of what exactly ‘reproductive isolation’ consists of. Mayr said that the mechanisms of reproductive isolation may be either pre-mating (where members of different species do not normally regard each other as potential mates) or post-mating (where they do mate, but the hybrids do not survive, or are sterile). In the case of lions and leopards, evidently the reproductive isolating mechanisms are pre-mating, because normally they do not regard each other as potential mates, but these can break down if a male of one species and a female of the other are caged together, a case of making the best of a bad job, if you like. Their post-mating reproductive isolation, however, is incomplete: male lion-leopard hybrids are thought to be sterile, but the females are fertile.
So far so good. According to the Biological Species Concept, different species are defined by not usually forming hybrids with each other, for whatever reason, under natural conditions. But it is not so simple.
Consider leopards, again. They live not only in Africa and India, but also on the island of Sri Lanka, and throughout Southeast Asia, including the island of Java. The leopards of Sri Lanka and Java obviously do not interbreed with those of the mainland, because they are separated by water barriers. According to Ernst Mayr’s definition, species are ‘actually or potentially interbreeding natural populations’, and presumably island leopards are to be regarded as ‘potentially interbreeding’ with mainland ones. But how do we know? How could we possibly know?

© J. Dougherty
The closest relative of the lion and the leopard is the jaguar, which lives in South and Central America, and likewise doesn’t have the chance to interbreed with leopards (or with lions, for that matter), so again, the ‘potentially interbreeding’ criterion breaks down. I would ask, and it is legitimate to ask, why is the jaguar classified as a species separate from the African and mainland-Asian leopard, whereas the Sri Lankan and Javanese leopards are not?
In my opinion, ‘potentially interbreeding’, is, really, a phantom concept. The Biological Species Concept offers no guidance at all for deciding whether populations living in different areas are distinct species or not. As one example from my own experience, mammal specialists have had heated discussions over whether the American bison and the European bison are or are not different species, a particularly pointless exercise if one accepts the Biological Species Concept. It was as early as the 1960s that a few taxonomists began to worry about this, because they were starting to realise that there were quite a lot of cases where they really needed to know. Gilbert’s potoroo, from the south-west of Western Australia, is it, or is it not, a different species from the Long-nosed potoroo, from south-eastern Australia? This may sound like a piece of pedantry, but it is in fact not a trivial decision, because Gilbert’s potoroo is critically endangered, and if it is not really a distinct species then it is less of a worry.
It was a group working in the American Museum of Natural History, known as the New York Group and already getting a reputation for asking awkward questions, that was pushing most strongly for a resolution, and in 1983, one of them, the ornithologist Joel Cracraft, proposed to replace the Biological Species Concept altogether and define a species ‘The smallest cluster of individual organisms within which there is a parental pattern of ancestry and descent, and that is diagnosably distinct from other such clusters by a unique combination of fixed character states’. What this means is that a species is a population or group of populations (this is the ‘parental pattern of ancestry and descent’ bit) which can be distinguished 100% from any other (this is the ‘diagnosably distinct’ bit). This concept of species is called the Phylogenetic Species Concept.
Many biologists, myself included, I’m afraid, started off by disliking the Phylogenetic Species Concept, and hoped it would die a natural death. But it did not; in fact it spread because many biologists, including taxonomists, and at long last I too, realised that it provides an objective criterion, diagnosability, for all cases, which the old Biological Species Concept does not. It tells us, for example, that Sri Lankan and Javanese leopards are not distinct species, because they cannot be 100% distinguished from the leopards of the mainland, whereas the jaguar is a distinct species because it is 100% distinct from its relatives.

© P. Mays
Much taxonomy today depends on molecular genetics, DNA sequencing. At present, many molecular geneticists tend to distinguish species rather subjectively, if they differ ‘enough’, though what is meant by ‘enough difference’ varies from one study to another. The Phylogenetic Species Concept is of course excellently suited to DNA sequencing, and many species have been recognised by having consistent differences in DNA sequences (the diagnosability criterion).
The molecular revolution has also taught us something important about species, that they do in fact interbreed under natural conditions, to a much greater extent than we had thought. We know this, because there is a form of DNA, mitochondrial DNA, that is inherited not from both parents, but from the mother alone; it is passed solely down the female line (with apparently few exceptions). And we now know quite a number of cases where a population of one species has the mitochondrial DNA of a different, related species.
Here is a nice example. The common deer species of the eastern United States is the white-tailed deer. In the west, it is replaced by the mule deer, and in the middle they live side-by-side in the same habitats. On a large ranch in West Texas, there are herds of both species, and they have the same mitochondrial DNA! There has been some dispute in the past over whose mitochondrial DNA it actually is, but it now appears that it is that of the mule deer. We imagine that, at some time in the past, some white-tailed bucks, unable to find does of their own species, ‘made the best of a bad job’ and drove off some mule deer bucks and mated with mule deer does. Hybrids were born, and in the next generation more white-tail bucks came over and mated with them. The hybrids are now three-quarters white-tail, and one-quarter mule deer, but of course they still had the mitochondrial DNA of their mule deer grandmothers. In a few more generations, they would come to totally resemble white-tailed deer, the only legacy of their original maternal heritage being their mitochondrial DNA.
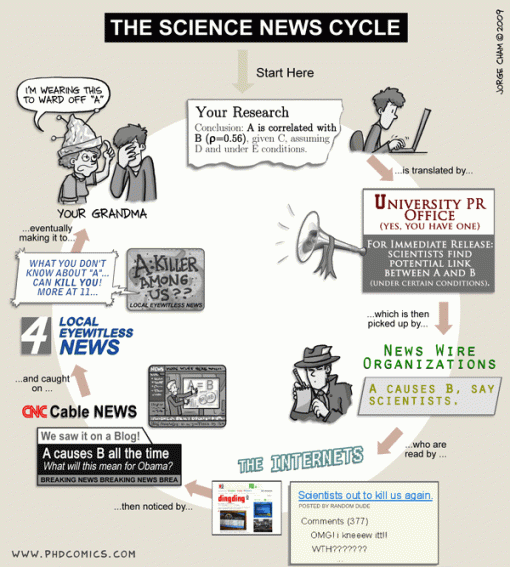











 We do a lot in our lab to get our research results out to a wider community than just scientists – this blog is just one example of how we do that. But of course, we rely on the regular media (television, newspaper, radio) heavily to pick up our media releases (see
We do a lot in our lab to get our research results out to a wider community than just scientists – this blog is just one example of how we do that. But of course, we rely on the regular media (television, newspaper, radio) heavily to pick up our media releases (see 


 As mentioned, quite a long analysis of the state of sharks worldwide. Bottom line? Well, as most of you might already know sharks aren’t doing too well worldwide, with around 52 % listed on the IUCN’s
As mentioned, quite a long analysis of the state of sharks worldwide. Bottom line? Well, as most of you might already know sharks aren’t doing too well worldwide, with around 52 % listed on the IUCN’s  In some ways this sort of goes against the notion that sharks are inherently more extinction-prone than other fish – a common motherhood statement seen at the beginning of almost all papers dealing with shark threats. What it does say though is that because sharks are on average larger and less fecund than your average fish, they tend to bounce back from declines more slowly, so they are more susceptible to rapid environmental change than your average fish. Guess what? We’re changing the environment pretty rapidly.
In some ways this sort of goes against the notion that sharks are inherently more extinction-prone than other fish – a common motherhood statement seen at the beginning of almost all papers dealing with shark threats. What it does say though is that because sharks are on average larger and less fecund than your average fish, they tend to bounce back from declines more slowly, so they are more susceptible to rapid environmental change than your average fish. Guess what? We’re changing the environment pretty rapidly.

































 I love these sorts of experiments. Ecology (and considering conservation ecology a special subset of the larger discipline) is a messy business, mainly because ecosystems are complex, non-linear, emergent, interactive, stochastic and meta-stable entities that are just plain difficult to manipulate experimentally. Therefore, making inference of complex ecological processes tends to be enhanced when the simplest components are isolated.
I love these sorts of experiments. Ecology (and considering conservation ecology a special subset of the larger discipline) is a messy business, mainly because ecosystems are complex, non-linear, emergent, interactive, stochastic and meta-stable entities that are just plain difficult to manipulate experimentally. Therefore, making inference of complex ecological processes tends to be enhanced when the simplest components are isolated.









 I just returned from a week-long scientific mission in China sponsored by the
I just returned from a week-long scientific mission in China sponsored by the 




 Ah, it doesn’t go away, does it? Or at least, we won’t let it.
Ah, it doesn’t go away, does it? Or at least, we won’t let it. I’m going to do a double review here of two papers currently online in
I’m going to do a double review here of two papers currently online in 



 Species, in the words of the great evolutionary biologist
Species, in the words of the great evolutionary biologist 

 A good friend and colleague of mine, Dr.
A good friend and colleague of mine, Dr. 

 Although buffalo in Australia experienced two major periods of population reduction since their introduction, a small proportion (estimated at ~ 20 %) escaped the BTEC reduction in the eastern part of its north Australian range. BTEC did not operate with uniformity across the entire range of buffalo, concentrating its destocking efforts in a general area from the western coast of the Northern Territory to west of the Mann River in Arnhem Land, and south roughly to Kakadu National Park’s southern border. Coincidently and not surprisingly, it is in this area that we observe most migration activity.
Although buffalo in Australia experienced two major periods of population reduction since their introduction, a small proportion (estimated at ~ 20 %) escaped the BTEC reduction in the eastern part of its north Australian range. BTEC did not operate with uniformity across the entire range of buffalo, concentrating its destocking efforts in a general area from the western coast of the Northern Territory to west of the Mann River in Arnhem Land, and south roughly to Kakadu National Park’s southern border. Coincidently and not surprisingly, it is in this area that we observe most migration activity.

